The "Other" Carbon Problem — Ocean Acidification
By Dave Cohen
19 August, 2010
Decline of the Empire
Humankind's assault on the oceans continues apace. A short time ago, we considered the loss of 40% of the phytoplankton in the oceans since 1950. In my post How We Wrecked The Oceans, marine ecologist Jeremy Jackson explains why he believes the sea will be devoid of fish and other large marine organisms sometime in the 2040s. And now comes the "other" carbon problem—acidification of the oceans.
As we burn fossil fuels, carbon dioxide (CO2) is released into the atmosphere. Everyone knows that part, but what they often don't know is that the oceans act as a enormous carbon "sink" which absorbs as much as 1/3rd of the released carbon dioxide. So the CO2 is no longer acting as a greenhouse gas in the atmosphere, which sounds good, but unfortunately, we have shifted the problem of dealing with the excess gas from the air to the oceans. Through some fairly simple chemistry, the oceans are becoming more acidic as a result. In other words, through a natural process, the ocean becomes a giant waste dump for our fossil fuel emissions.
A recent article in Scientific American called How Acidification Threatens Oceans from the Inside Out (subscription required) explains the deal—
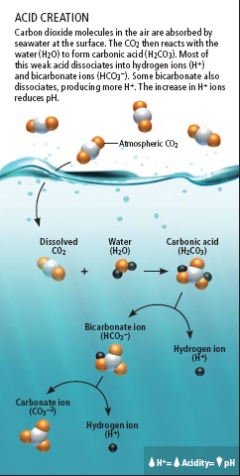
The ocean’s interaction with CO2 mitigates some climate effects of the gas. The atmospheric CO2 concentration is almost 390 parts per million (ppm), but it would be even higher if the oceans didn’t soak up 30 million tons of the gas every day. The world’s seas have absorbed roughly one third of all CO2 released by human activities. This “sink” reduces global warming—but at the expense of acidifying the sea. Robert H. Byrne of the University of South Florida has shown that in just the past 15 years, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska. Across the planet, the average pH of the ocean’s surface layer has declined 0.12 unit, to approximately 8.1, since the beginning of the industrial revolution.
That change may not sound like much, but because the pH scale is logarithmic, it equates to a 30 percent increase in acidity. Values of pH measure hydrogen ions (H+) in solution. A value of 7.0 is neutral; lower values are increasingly acidic, and higher values are basic. Although 8.1 is mildly basic, the declining trend constitutes acidification. Marine life has not experienced such a rapid shift in millions of years. And paleontology studies show that comparable changes in the past were linked to widespread loss of sea life.
[The pH of a solution is determined by the concentration of hydrogen ions (H+) in it. A value of 7.0 is neutral; lower is acidic; higher is basic. “Acidification” means a drop in value, anywhere along the scale.]
Thus we have increased the acidity of the oceans by an astonishing 30% since the Industrial Revolution began. Intuition tells us that such a large change occurring in basically no time at all (on geological timescales) can't be good for life in the oceans, and it is not. Recently the National Research Council (NRC) issued a report on the growing acidification problem, which was described in Science Daily's Carbon Dioxide Emissions Causing Ocean Acidification to Progress a Unprecedented Rate—
Studies on a number of marine organisms have shown that lowering seawater pH with CO2 affects biological processes, such as photosynthesis, nutrient acquisition, growth, reproduction, and individual survival depending upon the amount of acidification and the species tested, the committee found. For example, some of the strongest evidence of the potential effects of ocean acidification on marine ecosystems comes from experiments on organisms with calcium carbonate shells and skeletons.
The results showed decreases in shell and skeletal growth in a range of marine organisms, including reef-building corals, commercially important mollusks such as oysters and mussels, and several types of plankton at the base of marine food webs.
The ability of various marine organisms to acclimate or adapt to ocean acidification is unknown, but existing data suggest that there will be ecological winners and losers, leading to shifts in the composition and functioning of many marine ecosystems, the committee said. Such ecosystem changes could threaten coral reefs, fisheries, protected species, and other natural resources.
Although changes in ocean chemistry caused by increasing atmospheric CO2 can be determined, not enough information exists to assess the social or economic effects of ocean acidification, much less develop plans to mitigate or adapt to them, the committee noted.
So what can we do about all this? Not a Damn Thing. As long as we continue to burn fossil fuels, thus emitting excess CO2 into the atmosphere, the oceans will absorb about 30-35% of it. Science Daily sums up the NRC's conclusion that the ability of various marine organisms to acclimate or adapt to ocean acidification is unknown. If you've read a lot of these scientific reports by committee, as I have, you know that the word "unknown" is bureaucratic code standing for we're fucked, but the exact extent to which we're fucked is unknown.
Consider these remarks from the Scientific America article cited above. The authors are talking about the possibility of adaptation by marine organisms—
Lab experiments persist for weeks to months. Climate change occurs over decades and centuries. Some species could adapt, especially if they have a short reproductive cycle. Each time an animal reproduces, genetic mutations can arise in the offspring that might help the next generation adjust to new circumstances. Ninety years—the predicted time frame for pH to decline by 0.3 to 0.5 unit—is extremely short, however, for genetic adaptation by species that reproduce at relatively slow rates and that may already be stressed by the 30 percent pH decrease. Species extinctions often result from slow declines over centuries or more; a decline of just 1 percent of individuals per generation could cause extinction in less than a century.
Alarmingly, the pH drop observed so far and the predicted trajectory under current emissions trends are 100 times faster than any changes in prior millennia. Left unchecked, CO2 levels will create a very different ocean, one never experienced by modern species. Adaptation is even more unlikely because the effects of acidification, and the other struggles creatures face, interact. For example, increased CO2 levels can narrow the temperature range in which an individual can survive. We already see such constraints on corals and some algae, which become heat-stressed at lower temperatures than normal if exposed to higher CO2.
The only Good News here is that current CO2 emissions trends are very unlikely to continue for 90, 40, or even the next 20 years. Even the next 10 years is looking iffy.
Although there is some uncertainty about the timing & effects of ocean acidification, you know and I know that for this and other reasons, the Earth's oceans will likely be toast by 2050. And you know and I know that when something is toast, especially a Really Big Thing like the world's oceans, we Homo sapiens like to make a video about it with the word "challenge" in the title.
Here it is, narrated by Sigourney Weaver and produced by the National Resource Defense Council. It's very good.

